【协和医学杂志】人脐带间充质干细胞在膝骨关节炎治疗中的研究进展
时间:2025-03-03 12:17:25 热度:37.1℃ 作者:网络
综 述
膝骨关节炎(KOA)是一种膝关节退行性疾病,其病理特征为滑膜炎症、骨质增生、骨赘形成及关节软骨的逐渐丢失,进而引发关节疼痛、肿胀、畸形和活动受限等临床症状[1-2]。若长期不治疗,KOA可能会造成关节功能丧失,严重影响患者的生活质量[3-4]。目前临床常用的治疗方式包括非药物治疗、药物治疗、手术治疗等[5-7]。上述治疗手段主要致力于缓解疼痛、改善关节功能以及延缓KOA的进展,并不能彻底修复关节软骨的损伤,且患者可能需要面临多次手术治疗,无疑加重了其经济负担[2-3]。
间充质干细胞(MSCs)作为再生医学的热门研究方向,已在治疗缺血性心力衰竭、皮肤创伤、移植排异、糖尿病、肝病以及神经损伤等多种疾病中显示出较大潜力[8-13]。MSCs来源广泛,在骨髓、脂肪组织、脐带、滑膜、滑液、牙髓、毛囊、扁桃体和胎盘等多种组织中均有发现[3,7,14-17]。其中,人脐带间充质干细胞(hUC-MSCs)因无创、便利的获取方式以及较少的伦理争议,被认为是首选的MSCs来源[10,18-20]。本文将阐述hUC-MSCs在KOA治疗方面的作用原理、干预方案、效果及安全性,以期为KOA临床治疗提供更多可行方案。
1 hUC-MSCs在KOA治疗中的作用原理及应用基础
1.1 作用原理
1.1.1 促进软骨再生
促进软骨再生是hUC-MSCs区别于其他传统治疗方式的关键。已知的具体机制可概括如下:
1 通过分化为软骨细胞,直接参与软骨的合成[21-24];
2 通过激活Wnt/β-catenin信号通路,增强软骨细胞的增殖与分化能力[25];
3 分泌生长因子和细胞因子,如血管内皮生长因子(VEGF)、转化生长因子β(TGF-β)等,促进软骨细胞的增殖和分化,增加软骨基质的合成,同时增加Ⅱ型胶原蛋白(Col-Ⅱ)和降低基质金属蛋白酶13(MMP13)及含Ⅰ型血小板结合蛋白基序的解聚蛋白样金属蛋白酶-5(ADAMTS-5)的合成,维持软骨内环境平衡,以减少软骨损伤[23-31];
4 上调软骨形成相关基因表达,如X型胶原蛋白(Col-Ⅹ)、B细胞淋巴瘤-2基因(BCL-2)和TSG-6基因(tumor necrosis factor alpha-stimulated gene-6)的表达,促进软骨细胞存活[22];
5 通过抑制mTOR途径和激活自噬,减少软骨细胞凋亡[30]。
上述机制协同作用,可有效增加软骨厚度,促进软骨基质合成与沉积,同时维持软骨微环境的稳态,从根本上解决了因软骨丢失而引起的KOA问题。
1.1.2 调节炎症反应
调节炎症反应是延缓KOA进展的基础[22,32],已知的具体机制包括:
1 调节炎症因子发挥抗炎作用:一方面降低促炎因子如一氧化氮(NO)、白细胞介素(IL)-6、IL-1β和肿瘤坏死因子-α(TNF-α)的水平,另一方面分泌IL-10和IL-4等抗炎因子,有效减轻关节炎症和软骨破坏程度[27,31-32];
2 促进巨噬细胞极化:通过调节巨噬细胞的极化状态,促进巨噬细胞从促炎向抗炎状态转变,从而抑制炎症[26];
3 调节免疫反应:hUC-MSCs可分泌吲哚胺2,3-双加氧酶(IDO),抑制T细胞增殖,调节免疫反应,进一步减轻炎症反应[28,33]。
通过调控免疫反应和抗炎途径,hUC-MSCs显著降低了KOA的炎症水平,有效缓解关节软骨破坏进程。
1.2 应用基础
目前,hUC-MSCs关节内注射治疗已在大鼠、小鼠、兔、绵羊等动物模型中进行了在体实验。实验结果一致证明,hUC-MSCs能够减轻KOA引起的软骨破坏和炎症反应,促进软骨缺损修复,并延缓KOA进展,同时展现出良好的安全性和有效性[21-22,25-27,31-32]。具体而言,通过向KOA大鼠模型膝关节中注射hUC-MSCs,发现hUC-MSCs不仅可有效减轻术后膝关节炎症反应,还可显著提升关节的活动能力[22,25-26,31]。
CT和X线检查结果显示,hUC-MSCs可显著改善软骨下骨的密度和形态,减少骨赘形成,缓解关节腔狭窄情况[25,31,34-35]。对手术部位的软骨进行取材染色发现,hUC-MSCs治疗组的软骨结构更完整,软骨细胞排列更整齐,软骨基质染色强度增强,且软骨厚度较术前增加了125%[25-26,31]。这表明hUC-MSCs治疗不仅可增加关节软骨厚度,减缓关节软骨破坏,还改善了关节腔的整体环境。
此外,Tong等[22]通过对比单次与多次注射hUC-MSCs的效果发现,多次注射可更显著地改善大鼠软骨侵蚀并有效减缓疾病进展。同时,研究人员对hUC-MSCs与透明质酸(HA)、氧化石墨烯颗粒润滑剂联合应用发现,相较于单独使用hUC-MSCs,联合应用能更有效地促进新西兰兔和绵羊膝关节的软骨修复[21,32,36]。且这些研究均未报告与治疗相关的严重不良反应或死亡事件,进一步显示使用hUC-MSCs治疗的安全性。
2 hUC-MSCs在KOA治疗中的临床研究
2.1 临床研究设计类型及特征
笔者共检索到13项关于hUC-MSCs治疗KOA的临床研究,患者年龄18~67岁,无未成年人参与。膝关节病损情况以中重度为主,主要纳入Kellgren Lawrence(KL)2~4级和国际软骨修复协会(ICRS)评分Ⅲ~Ⅳ级的患者,少量纳入KL 1级的KOA轻度患者。随访时间从3个月至5年不等,可有效观察hUC-MSCs治疗的疗效及不良反应(表1)。
表1 hUC-MSCs在KOA治疗中的临床应用研究
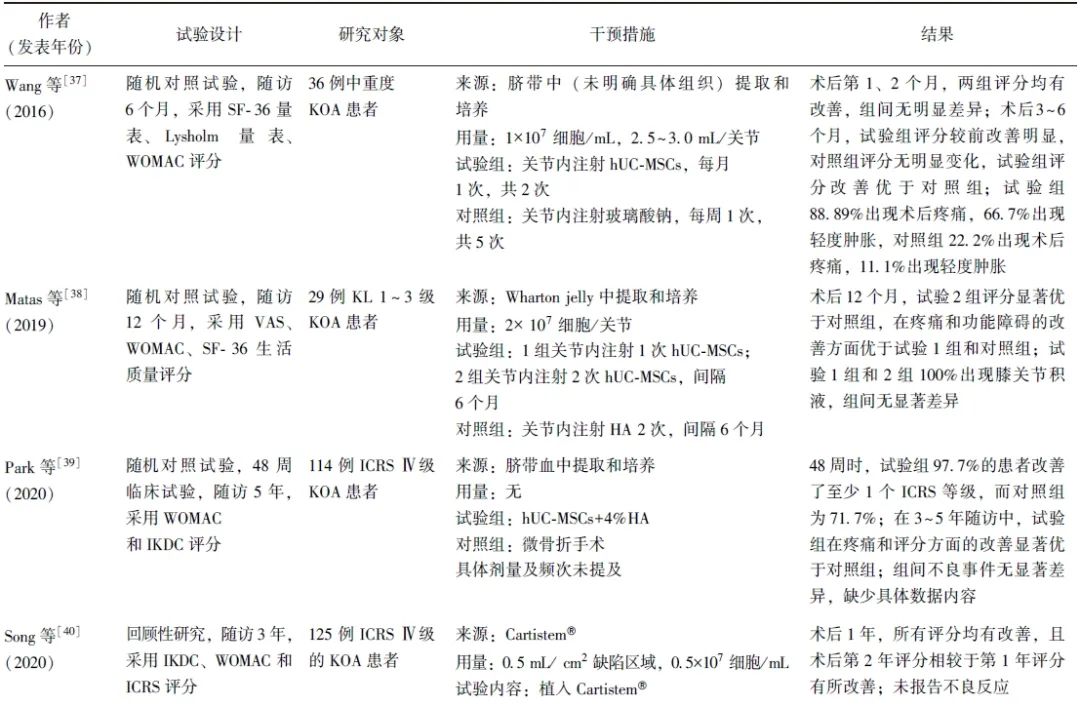
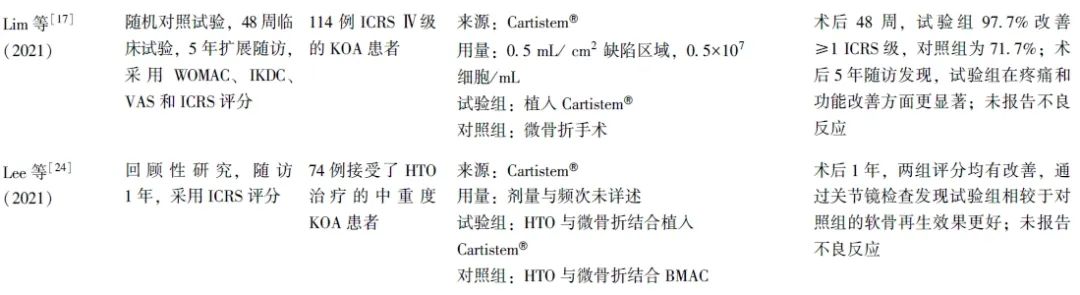
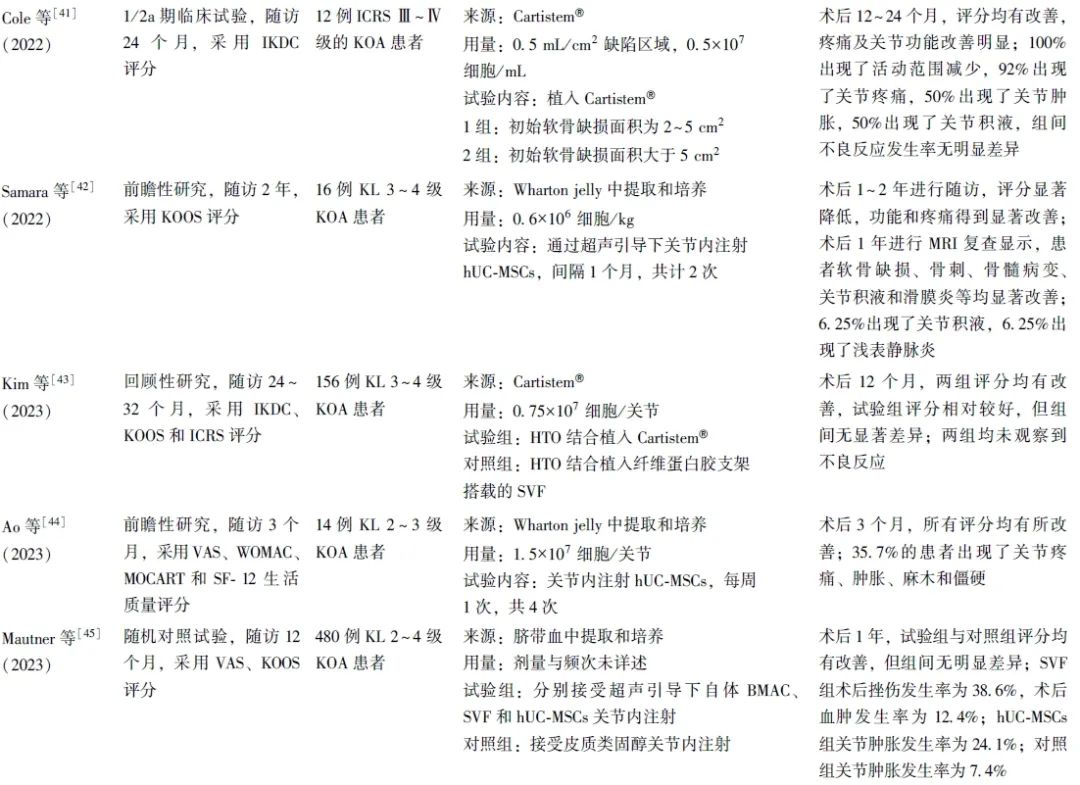

2.2 hUC-MSCs的临床干预方案
2.2.1 细胞来源
研究使用的hUC-MSCs来源分为自行提取/培养和成品商品2种类型。自行培养/提取的hUC-MSCs又可分为脐带血来源和Wharton jelly组织来源。成品商品主要是Cartistem®,由Medipost公司生产的含有hUC-MSCs(7.5×106细胞)和4%HA的成品。
2.2.2 细胞使用方式
研究多采用开放性手术制造关节软骨缺损,并植入hUC-MSCs,部分研究采用直接或超声引导下关节内注射hUC-MSCs,还有部分研究采用hUC-MSCs植入与胫骨高位截骨术(HTO)及微骨折手术联合治疗方案。
2.2.3 细胞剂量和频次
hUC-MSCs植入量多控制于(0.75~4.5)×107细胞/关节,也有研究采用较高剂量的hUC-MSCs(1×108细胞/关节)进行注射。关节内注射疗法更为简便,且可重复性更高,可进行多次注射,临床多采用1~6个月间隔注射,也有研究采用间隔1周,共计4次的注射方案。具体注射剂量及频次有待继续研究优化,以寻找最佳的注射剂量及频次,为患者提供个性化的治疗方案。
2.3 临床疗效
研究多采用视觉模拟评分(VAS)、西安大略和麦克马斯特大学骨关节炎指数(WOMAC)、国际膝关节评分委员会膝关节主观功能评分量表 (IKDC)及膝关节损伤和骨关节炎结果评分(KOOS)对关节的疼痛程度、功能障碍及临床疗效进行评估,并通过关节镜下国际软骨修复学会(ICRS)评分及软骨修复组织磁共振观察(MOCART)评分评估关节软骨的修复情况,也有部分研究采用SF-12(short form-12)生活质量评分、SF-36量表、Lysholm量表对KOA患者的生活质量进行评估。
结果发现,hUC-MSCs治疗可显著改善患者的膝关节宏观评分和疼痛指数。关节镜下观察显示,患者软骨修复等级也得到了显著提升。且无论单独应用还是与HA、HTO或微骨折手术联合应用,hUC-MSCs在改善膝关节功能和缓解疼痛方面均优于传统手术方法。
进一步的剂量效应研究结果显示,低剂量和中剂量组在疼痛缓解和功能改善方面表现出相似效果,而高剂量组的效果相对较低,提示hUC-MSCs的治疗效果可能与其浓度剂量相关[47]。
2.4 不良反应
多项研究均报告患者术后存在轻微不良反应,如疼痛、肿胀、麻木、活动范围减少、关节积液、血肿和浅表静脉炎等[37-38,41-42,44-45,47]。这些不良反应通常在注射后3~12 h内出现,具有自限性(可在3 d内缓解),部分患者需通过相关的干预措施进行控制[37]。关于移植及合并HTO、微骨折手术的研究,由于手术创伤较大,患者难以区分疼痛的具体来源,且术后通常采取制动措施,在一定程度上减少了关节腔的挤压,从而影响了对于不良反应的客观观察[24,43,46]。Matas等[47]研究显示,高剂量组患者普遍出现关节疼痛和肿胀,而低剂量组仅有40%的患者出现类似症状,提示不良反应的发生可能与注射的hUC-MSCs浓度相关。关于这些不良反应的发生机制,有研究推测可能与注射行为本身有关,也可能与关节内干细胞诱导的轻度滑膜炎症有关[38,41,45]。但目前仍缺乏充分的证据证实不良反应发生的具体机制,有待进一步探索。
2.5 不良反应的处理
在临床研究中,为了应对治疗相关不良反应,通常采取如下管理措施:预防性注射地塞米松、使用止痛药物或进行关节穿刺抽液[37-38]。实践证明,此类方法可有效控制症状,且在术后6个月至5年的随访过程中未观察到与治疗相关的死亡或严重事件,提示该疗法较安全[18,48]。未来,可考虑进一步优化治疗方式和hUC-MSCs浓度,以寻求最佳治疗效果与最小不良反应,为患者提供更加优质的治疗体验。
3 小结与展望
综上所述,应用hUC-MSCs治疗KOA可显著改善患者关节功能、缓解疼痛和促进软骨修复,且显示出良好的安全性。对于轻中度KOA患者,hUC-MSCs注射疗法相较于传统疗法具有更小的创伤、更高的经济可行性和更好的疗效[6,17,37]。
对于膝关节病变严重,如KL 3~4级或ICRS Ⅲ~Ⅳ级的患者,采用HTO联合hUC-MSCs移植可能提供更优的治疗效果[24,40,46]。然而目前的研究证据并不充分,为提高研究质量,建议未来延长随访时间、扩大样本量,开展更多的随机对照试验,以增强证据质量和可靠性。
目前,我国已成功构建了临床级hUC-MSCs资源库,包括种子细胞库、主细胞库和工作细胞库,能够为干细胞研究提供质量可控的临床级hUC-MSCs资源[49]。然而,未来若要将hUC-MSCs治疗KOA广泛应用于临床实践,仍需解决一些关键问题。
首先,细胞扩增问题亟待解决。尽管已有研究表明微载体-生物反应器系统可提高hUC-MSCs的扩增效率并延缓衰老[50-51],但目前的扩增速度仍未能满足大规模临床应用需求。未来研究需聚焦于开发更高效、稳定的扩增技术,以确保细胞数量和质量满足高标准的临床应用。
其次,需明确hUC-MSCs在KOA治疗中的作用机制,并探索不良反应的成因,这对于优化治疗方案和提升患者治疗体验至关重要。
再次,由于KOA患者在不同阶段病情差异明显,需进行更多的临床试验,以确定最佳治疗剂量和频率,实现个性化治疗。
最后,为确保hUC-MSCs的临床应用,必须建立完整的产品生产和质量控制流程。同时,还需警惕商业化过程中的利益冲突,确保治疗推广严格基于科学证据。
期待通过上述问题的不断解决,进一步提高hUC-MSCs治疗的有效性和安全性,为KOA患者带来更多福音。
参考文献
[1]龚恒, 陈连旭, 黄斌. 关节内注射间充质干细胞治疗退行性膝骨关节炎临床研究进展[J]. 中国运动医学杂志, 2020, 39(9): 746-750.
[2]Wu Q L, Wu Z Q, Lu Z F. Clinical efficacy and safety of the combination of mesenchymal stem cells and scaffolds in the treatment of knee osteoarthritis: protocol for systematic review and meta-analysis[J]. Medicine (Baltimore), 2022, 101(43): e31638.
[3]Xu X, Xu L M, Xia J, et al. Harnessing knee joint resident mesenchymal stem cells in cartilage tissue engineering[J]. Acta Biomater, 2023, 168: 372-387.
[4]Chu H S, Zhang S Y, Zhang Z L, et al. Comparison studies identify mesenchymal stromal cells with potent regenerative activity in osteoarthritis treatment[J]. NPJ Regen Med, 2024, 9(1): 14.
[5]中华医学会骨科学分会关节外科学组, 解放军总医院第四医学中心骨科医学部, 国家骨科与运动康复临床医学研究中心. 中国膝骨关节炎非手术治疗专家共识(2023年版)[J]. 中华关节外科杂志(电子版), 2024, 18(2): 151-159.
[6]Suh K, Cole B J, Gomoll A, et al. Cost effectiveness of allogeneic umbilical cord blood-derived mesenchymal stem cells in patients with knee osteoarthritis[J]. Appl Health Econ Health Policy, 2023, 21(1): 141-152.
[7]Li Y F, Zhang Y N, Wang H, et al. Dental pulp mesenchymal stem cells attenuate limb ischemia via promoting capillary proliferation and collateral development in a preclinical model[J]. Stem Cells Int, 2021, 2021: 5585255.
[8]Li B, Cheng Y, Yu S Y, et al. Human umbilical cord-derived mesenchymal stem cell therapy ameliorates nonal-coholic fatty liver disease in obese type 2 diabetic mice[J]. Stem Cells Int, 2019, 2019: 8628027.
[9]Arderiu G, Civit-Urgell A, Badimon L. Adipose-derived stem cells to treat ischemic diseases: the case of peripheral artery disease[J]. Int J Mol Sci, 2023, 24(23): 16752.
[10]Ryu D J, Jeon Y S, Park J S, et al. Comparison of bone marrow aspirate concentrate and allogenic human umbilical cord blood derived mesenchymal stem cell implantation on chondral defect of knee: assessment of clinical and magnetic resonance imaging outcomes at 2-year follow-up[J]. Cell Transplant, 2020, 29: 963689720943581.
[11]Li Z T, Wang Y M, Wang H, et al. Self-assembled DNA composite-engineered mesenchymal stem cells for improved skin-wound repair[J]. Small, 2024, 20(31): 2310241.
[12]Xie H C, Wang Z G, Feng Y H, et al. Bone marrow mesenchymal stem cells repress renal transplant immune rejection by facilitating the APRIL phosphorylation to induce regulation B cell production[J]. Physiol Genomics, 2023, 55(2): 90-100.
[13]Yalçin M B, Bora E S, Erdogan M A, et al. The effect of adipose-derived mesenchymal stem cells on peripheral nerve damage in a rodent model[J]. J Clin Med, 2023, 12(19): 6411.
[14]Fan M Q, Zhang J W, Zhou L, et al. Intra-articular injection of placental mesenchymal stromal cells ameliorates pain and cartilage anabolism/catabolism in knee osteoarthritis[J]. Front Pharmacol, 2022, 13: 983850.
[15]Sadri B, Tamimi A, Nouraein S, et al. Clinical and laboratory findings following transplantation of allogeneic adipose-derived mesenchymal stromal cells in knee osteoarthritis, a brief report[J]. Connect Tissue Res, 2022, 63(6): 663-674.
[16]He S, Zhang J, Chen W J, et al. Umbilical cord mesenchymal stem cells promote the repair of trochlear groove reconstruction in dogs[J]. Front Vet Sci, 2022, 9: 922390.
[17]Lim H C, Park Y B, Ha C W, et al. Allogeneic umbilical cord blood-derived mesenchymal stem cell implantation versus microfracture for large, full-thickness cartilage defects in older patients: a multicenter randomized clinical trial and extended 5-year clinical follow-up[J]. Orthop J Sports Med, 2021, 9(1): 2325967120973052.
[18]Kim K I, Lee W S, Kim J H, et al. Safety and efficacy of the intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritic knee: a 5-year follow-up study[J]. Stem Cells Transl Med, 2022, 11(6): 586-596.
[19]BANERJI B. Allogenic human umbilical cord derived mesenchymal stem cells for managing knee osteoarthritis in human-a clinical trial[J]. J Regen Biol Med, 2021, 3(5): 1-15.
[20]Hori A, Takahashi A, Miharu Y, et al. Superior migration ability of umbilical cord-derived mesenchymal stromal cells (MSCs) toward activated lymphocytes in comparison with those of bone marrow and adipose-derived MSCs[J]. Front Cell Dev Biol, 2024, 12: 1329218.
[21]Lubis A M T, Situmeang A, Canintika A F, et al. Intra-articular injection of human umbilical cord-derived mesen-chymal stem cells in sheep models of meniscectomy-induced osteoarthritis: an experimental study[J]. Orthop J Sports Med, 2023, 11(S2): 2325967121S00913.
[22]Tong W, Zhang X G, Zhang Q, et al. Multiple umbilical cord-derived MSCs administrations attenuate rat osteoarthritis progression via preserving articular cartilage superficial layer cells and inhibiting synovitis[J]. J Orthop Translat, 2020, 23: 21-28.
[23]Tian H, Tong W, Zhang X, et al. Umbilical cord derived mesenchymal stem cells attenuate rodent Osteoarthritis progression via preserving articular cartilage superficial layer cells and inhibiting synovitis[J]. Osteoarthritis Cartilage, 2020, 28(S1): S513-S514.
[24]Lee N H, Na S M, Ahn H W, et al. Allogenic human umbilical cord blood-derived mesenchymal stem cells are more effective than bone marrow aspiration concentrate for cartilage regeneration after high tibial osteotomy in medial unicompartmental osteoarthritis of knee[J]. Arthroscopy, 2021, 37(8): 2521-2530.
[25]Zhou Y, Zhao Y J, Wu Y J, et al. Human umbilical cord mesenchymal stem cells alleviate rat knee osteoarthritis via activating Wnt/β-catenin signaling pathway[J]. Curr Stem Cell Res Ther, 2024, 19(2): 234-244.
[26]Tang S J, Chen P H, Zhang H R, et al. Comparison of curative effect of human umbilical cord-derived mesenchymal stem cells and their small extracellular vesicles in treating osteoarthritis[J]. Int J Nanomedicine, 2021, 16: 8185-8202.
[27]Perry J, Roelofs A J, Mennan C, et al. Human mesenchymal stromal cells enhance cartilage healing in a murine joint surface injury model[J]. Cells, 2021, 10(8): 1999.
[28]Perry J, McCarthy H S, Bou-Gharios G, et al. Injected human umbilical cord-derived mesenchymal stromal cells do not appear to elicit an inflammatory response in a murine model of osteoarthritis[J]. Osteoarthr Cartil Open, 2020, 2(2): 100044.
[29]Ju Y, Yi L X, Li C, et al. Comparison of biological characteristics of human adipose- and umbilical cord- derived mesenchymal stem cells and their effects on delaying the progression of osteoarthritis in a rat model[J]. Acta Histochem, 2022, 124(6): 151911.
[30]Bie Y N, Chen Q Q, Xu J H, et al. Human umbilical-cord-derived mesenchymal stem cells in combination with rapamycin reduce cartilage degradation via inhibition of the AKT/mTOR signaling pathway[J]. Immunopharmacol Immunotoxicol, 2023, 45(5): 549-557.
[31]Pan X, Li X F, Zhang L, et al. Umbilical cord mesenchymal stem cells relieve osteoarthritis in rats through immunoregulation and inhibition of chondrocyte apoptosis[J]. Sci Rep, 2023, 13(1): 14975.
[32]Xing D, Wu J, Wang B, et al. Intra-articular delivery of umbilical cord-derived mesenchymal stem cells temporarily retard the progression of osteoarthritis in a rat model[J]. Int J Rheum Dis, 2020, 23(6): 778-787.
[33]Nativel F, Smith A, Marquis M, et al. Intra-articular injection of mesenchymal stromal cell encapsulated in micro-molded alginate particles for the treatment of post-traumatic osteoarthritis: preliminary study in rabbit knee[J]. Osteoarthritis Cartilage, 2021, 29(S1): S84.
[34]Liu A F, Chen J X, Zhang J T, et al. Intra-articular injection of umbilical cord mesenchymal stem cells loaded with graphene oxide granular lubrication ameliorates inflammatory responses and osteoporosis of the subchondral bone in rabbits of modified papain-induced osteoarthritis[J]. Front Endocrinol (Lausanne), 2021, 12: 822294.
[35]Bertoni L, Branly T, Jacquet S, et al. Intra-Articular injection of 2 different dosages of autologous and allogeneic bone marrow- and umbilical cord-derived mesenchymal stem cells triggers a variable inflammatory response of the fetlock joint on 12 sound experimental horses[J]. Stem Cells Int, 2019, 2019: 9431894.
[36]Wu J L, Wong P C, Ho C W, et al. Human umbilical cord mesenchymal stem cells in combination with hyaluronic acid ameliorate the progression of knee osteoarthritis[J]. Appl Sci, 2021, 11(14): 6650.
[37]Wang Y L, Jin W X, Liu H Y, et al. Curative effect of human umbilical cord mesenchymal stem cells by intra-articular injection for degenerative knee osteoarthritis[J]. Chin J Reparative Reconstr Surg, 2016, 30(12): 1472-1477.
[38]Matas J, Orrego M, Amenabar D, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase Ⅰ/Ⅱ trial[J]. Stem Cells Transl Med, 2019, 8(3): 215-224.
[39]Park Y, Ha C, Kim J, et al. Allogeneic umbilical cord blood-derived mesenchymal stem cells versus microfracture for large full-thickness cartilage defects[J]. Cytotherapy, 2020, 22(S5): S29-S30.
[40]Song J S, Hong K T, Kim N M, et al. Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up[J]. Regen Ther, 2020, 14: 32-39.
[41]Cole B J, Kaiser J T, Wagner K R, et al. Safety of an allogeneic, human, umbilical cord blood-derived mesenchymal stem cells-4% hyaluronate composite for cartilage repair in the knee[J]. J Cartilage Joint Preserv, 2022, 2(1): 100037.
[42]Samara O, Jafar H, Hamdan M, et al. Ultrasound-guided intra-articular injection of expanded umbilical cord mesenchymal stem cells in knee osteoarthritis: a safety/efficacy study with MRI data[J]. Regen Med, 2022, 17(5): 299-312.
[43]Kim Y S, Suh D S, Tak D H, et al. Adipose-derived stromal vascular fractions are comparable with allogenic human umbilical cord blood-derived mesenchymal stem cells as a supplementary strategy of high tibial osteotomy for varus knee osteoarthritis[J]. Arthrosc Sports Med Rehabil, 2023, 5(3): e751-e764.
[44]Ao Y N, Duan J J, Xiong N, et al. Repeated intra-articular injections of umbilical cord-derived mesenchymal stem cells for knee osteoarthritis: a phase Ⅰ, single-arm study[J]. BMC Musculoskelet Disord, 2023, 24(1): 488.
[45]Mautner K, Gottschalk M, Boden S D, et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: a randomized phase 3 trial[J]. Nat Med, 2023, 29(12): 3120-3126.
[46]Park Y B, Lee H J, Nam H C, et al. Allogeneic umbilical cord-blood-derived mesenchymal stem cells and hyaluronate composite combined with high tibial osteotomy for medial knee osteoarthritis with Full-Thickness cartilage defects[J]. Medicina (Kaunas), 2023, 59(1): 148.
[47]Matas J, García C, Poblete D, et al. A phase I dose-escalation clinical trial to assess the safety and efficacy of umbilical cord-derived mesenchymal stromal cells in knee osteoarthritis[J]. Stem Cells Transl Med, 2024, 13(3): 193-203.
[48]Md Yusoff B A H B, Mohd Don A F B, Mohamad N B, et al. ChondrogenTM injection for knee osteoarthritis using stem cells from Wharton's Jelly[J]. Sains Malaysiana, 2023, 52(10): 2773-2784.
[49]赵庆辉, 白志慧, 贾文文, 等. 临床级人脐带间充质干细胞资源库的构建[J]. 国际生物医学工程杂志, 2021, 44(6): 454-459.
[50]Kurogi H, Takahashi A, Isogai M, et al. Umbilical cord derived mesenchymal stromal cells in microcarrier based industrial scale culture sustain the immune regulatory functions[J]. Biotechnol J, 2021, 16(6): 2000558.
[51]Wang X, Ouyang L M, Chen W X, et al. Efficient expansion and delayed senescence of hUC-MSCs by microcarrier-bioreactor system[J]. Stem Cell Res Ther, 2023, 14(1): 284.


