CGP热点关注|二甲双胍治疗糖尿病肾病的研究进展
时间:2023-11-29 22:29:43 热度:37.1℃ 作者:网络
CGP 本文来源
邓煜璇,黄学君,江妍霞. 二甲双胍治疗糖尿病肾病的研究进展[J]. 中国全科医学, 2024, 27(03): 262-267.
糖尿病肾病(DN)是糖尿病微血管病变常见的并发症之一,可降低糖尿病患者的生活质量,是造成终末期肾衰竭的主要病因。二甲双胍是治疗糖尿病的主要药物之一,在糖尿病肾病治疗中起至关重要的作用。近年来,研究发现二甲双胍不仅可以通过多种机制降低血糖,还可以阻止糖尿病肾病发展为终末期肾衰竭。多项研究发现二甲双胍对于治疗糖尿病肾病具有临床疗效,应根据肾小球滤过率评估患者药物安全性。
本文综述了二甲双胍治疗糖尿病肾病的药理作用和作用机制的研究结果,旨在深入了解二甲双胍对糖尿病肾病的治疗作用,为糖尿病肾病的治疗方案提供参考。
01 二甲双胍的药理作用
1.1 降糖作用
二甲双胍的使用可减少低血糖的发生及糖尿病相关死亡。二甲双胍主要的作用机制是抑制肝脏葡萄糖生成,二甲双胍通过抑制肝细胞线粒体电子传递链复合物,导致腺嘌呤核苷酸磷酸化电位受损,AMPK水平升高,通过AMPK激活来抑制肝糖异生。
二甲双胍也可以通过非APMK依赖性方式来抑制肝糖异生。MA等研究发现低剂量二甲双胍可以和早老素增强因子-2(Pen2)结合,进一步与溶酶体辅助蛋白1(ATP6AP1)形成复合物,通过与AMPK激活的溶酶体感应通路相交,发挥其保护作用。
1.2 其他作用
近年来二甲双胍对多种疾病具有一定的疗效,可降低T2DM的微血管并发症的发生,可用于肿瘤预防和复发,并且恢复多囊卵巢综合征(PCOS)的卵巢功能。
临床研究发现,二甲双胍的疗效取决于肿瘤分期,可提高局部晚期肿瘤患者的生存期,对于远处转移的患者无明显改善作用。
研究表明二甲双胍可有效提高非排卵性月经周期的肥胖和非肥胖PCOS患者的妊娠率和活产率。
二甲双胍还具有降低尿蛋白排泄和保护肾功能的作用,一项动物实验发现二甲双胍减轻糖尿病大鼠肾小球表面积、足细胞间隙增大的病理学改变,还可以通过下调富亮氨酸蛋白a2糖蛋白1(LRG1)和转化生长因子β1(TGF-β1)/激活素受体样激酶1(ALK1)。
1.3 不良反应
二甲双胍最常见的不良反应为胃肠道反应,最严重的不良反应为二甲双胍性乳酸酸中毒。研究发现二甲双胍的使用需根据肾脏功能来决定。在早期DN患者中,二甲双胍发挥重要作用,可减轻T2DM患者的血糖和各种并发症的严重程度。然而在晚期肾损害患者中,二甲双胍可能具有增加乳酸酸中毒的风险。临床上,乳酸酸中毒是一种可导致急性死亡的疾病,以动脉血乳酸水平>5.0 mmol/L(参考值为0.5~2.2 mmol/L),血浆pH值<7.35为主要特征。既往研究表明二甲双胍在参考剂量范围内可安全使用,但在估计肾小球滤过率(eGFR)<30 mL·min-1·(1.73 m2)-1时,会出现急性肾损伤和血浆二甲双胍累积的风险,这种情况需完善乳酸浓度试验和动脉血气分析。
02 DN的病理特征
DN以肾小球硬化和小管间质纤维化为特征,主要的发病机制与肾小球高滤过、氧化应激、自噬、脂质代谢有关。此外,DN的发生与肾脏的炎症反应相关,如巨噬细胞募集、核转录因子κB(NF-κB)信号转导、细胞因子释放,炎症信号通路通过信号转导促进肾小球硬化和肾小球基底膜增厚。当糖尿病患者长期处于高血糖状态,会导致ROS激活,并且促进晚期糖基化终产物(AGEs)生成,导致肾素-血管紧张素-醛固酮系统(RAAS)激活,进一步出现肾损伤。自噬属于保护肾脏功能的良性机制,但慢性高血糖的刺激会引起自噬的速率和通量出现显著的变化,从而出现DN损伤。DN损伤的关键特征还包括足细胞足突消失和突变,足细胞发生损伤会出现尿蛋白和eGFR降低。尿蛋白和eGFR常作为诊断DN的标准,但eGFR降低可能不出现尿蛋白排泄。
03 DN的诊断标准
DN是T2DM较常见的临床并发症之一,基于UACR和eGFR下降为主要诊断标准。
临床特征:UACR持续升高或eGFR下降,尿沉渣异常(蛋白尿、管型尿或血尿)。
诊断金标准为肾脏病理活检,但对于糖尿病患者而言不可行常规肾穿刺,可行UACR检测,在3~6个月内连续检测UACR,其中至少有2次出现持续性蛋白尿(UACR≥30 mg/g)。
根据尿白蛋白排泄率进行分期:
(1)DN 1期为糖尿病初期,其特征为GFR明显增加;
(2)DN 2期为临床前阶段,其特征为尿蛋白正常,GFR轻度升高;
(3)DN 3期为DN初期,其特征为持续微量白蛋白尿(20~200 μg/min),轻度高血压,GFR正常或轻度升高;
(4)DN 4期为临床DN期,其特征为蛋白尿增多(>200 μg/min),伴水肿、高血压和肾功能受损;
(5)DN 5期为尿毒症期,其特征为蛋白尿进一步升高(>300 mg/g),GFR严重下降,血肌酐升高。
04 二甲双胍治疗DN的分子机制
二甲双胍治疗DN的分子机制见图1,包括:提高Klotho蛋白水平改善DN、通过促进AMPK介导的通路保护肾脏功能、通过抑制NF-κB介导的通路保护肾脏功能、通过抑制AGEs形成保护肾脏功能、通过调控微小RNA(miRNA)表达保护肾脏功能。
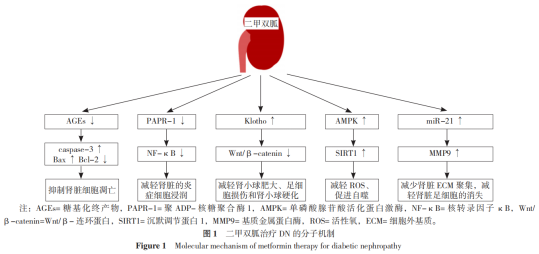
4.1 提高Klotho蛋白水平改善DN
二甲双胍作为主要治疗药物之一,通过调节Klotho蛋白水平保护肾脏的功能,研究发现二甲双胍可提高肾脏足细胞和Klotho蛋白的存活率,雷帕霉素(mTOR)是一种丝/苏氨酸蛋白激酶,参与生物合成、自噬和免疫介导过程。高糖可下调klotho表达,激活mTOR信号通路,加重糖尿病患者的肾脏损伤。SUN等指出TGF-β1可促进胶原蛋白分泌及诱导沉积,p38MAPK可促进细胞增殖,进一步研究表明二甲双胍可阻断Klotho依赖性TGF-β1/p38丝裂原活化蛋白激酶(MAPK)通路阻止肾脏纤维化,在Klotho蛋白介导的信号通路作用下,从而延缓糖尿病患者肾脏功能障碍的加重。
4.2 通过促进AMPK介导的通路保护肾脏功能
二甲双胍对DN有潜在保护作用,可以通过AMPK介导的信号通路缓解系膜细胞凋亡和足细胞损伤,保护肾脏免受氧化应激、炎症凋亡和上皮间质转化(EMT)。AMPK是由催化α-亚基和两个调节β和γ亚基组成,属于异源三聚体复合物,其介导生物能量代谢的调控,与糖脂代谢和蛋白质代谢有关。既往研究建立大鼠肾脏系膜细胞体外模型,采用基因沉默技术对AMPK/沉默调节蛋白1-叉头状转录因子O1(SIRT1-FoxO1)通路进行分析,发现AMPK可能参与调控DN的氧化应激和自噬。
4.3 通过抑制NF-κB介导的通路保护肾脏功能
NF-κB在体内参与很多生物反应,如细胞生长发育、免疫应答和炎症反应。DN患者肾脏病理可见大量炎症细胞浸润,主要以单核-巨噬细胞为主,伴有大量的炎症因子,如肿瘤坏死因子α(TNF-α)、白介素1(IL-1)和TGF-1β,这些因子经过级联放大反应加重肾脏损伤,形成蛋白尿,NF-κB可参与转录巨噬细胞,研究表明抑制NF-κB可减少炎症细胞对肾脏的损伤在高糖条件下会激活NF-κB,促进炎症因子损害肾脏。LING等研究发现白介素33(IL-33)属于IL-1家族,可显著抑制细胞生长发育,二甲双胍可能通过NF-κB通路降低血清IL-33浓度,抑制肾脏细胞凋亡,促进肾脏细胞增殖。
4.4 通过抑制AGEs形成保护肾脏功能
AGEs是慢性高血糖引起的病理性糖基化增加的结果,在高糖条件下AGEs累积会诱导足细胞损伤、TGF-β和系膜细胞凋亡的表达,促进肾脏衰老。AGEs会激活AGEs受体(AGEs receptor,RAGE),通过不同的信号转导通路促进氧化应激、内质网应激和纤维化,加速肾脏的结构和功能破坏。ISHIBASHI等报道AGEs上调肾近端小管RAGE mRNA水平,促进肾小管细胞损伤。细胞培养和动物培养观察到二甲双胍可能通过AMPK活化蛋白激酶抑制RAGE表达,从而抑制AGEs的形成和ROS的产生,出现抑制肾小管炎症反应和纤维化。
4.5 通过调控miRNA表达保护肾脏功能
二甲双胍直接作用于miR-21靶点并调节基质金属蛋白酶(MMP9)表达实现肾脏保护作用,MMP9以酶原的形式存在,其作用是降解胶原纤维、抑制ECM聚集、维持细胞膜的稳定性、清除自由基,并参与组织正常生长和受损部位愈合的过程,糖尿病患者由于长期处于高血糖水平,MMP9的表达受抑制,加重高血糖对肾脏的损害,二甲双胍作用于miR-21靶点,促进miR-21的表达,上调MMP9的水平,达到减少肾脏ECM聚集,减轻肾脏足细胞的消失。
T2DM及其各种并发症已经严重危害人类健康。我国糖尿病患病率与日俱增,由于人们对T2DM的认识不足,对疾病引起的健康状况未引起重视。DN是糖尿病的慢性并发症之一,最终会导致肾衰竭和尿毒症,二甲双胍是治疗糖尿病较常见的药物之一,有延缓肾脏组织损伤的作用,目前多数临床试验研究和动物实验均证实二甲双胍可通过各种信号通路治疗DN。然而,二甲双胍的具体不良反应仍需进一步研究,其是否同样适用于非DN还需进行探索。经过研究可以确定二甲双胍在DN治疗中起着至关重要的作用,为T2DM患者缓解肾脏病变提供了良好的方案。
参考文献
[1] WU Y L,DING Y P,TANAKA Y,et al. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention[J]. Int J Med Sci,2014,11(11):1185-1200.
[2] TAN S Y,MEI WONG J L,SIM Y J,et al. Type 1 and 2 diabetes mellitus:a review on current treatment approach and gene therapy as potential intervention[J]. Diabetes Metab Syndr,2019,13(1):364-372.
[3] MASSIMINO E,IZZO A,RICCARDI G,et al. The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes:current evidence and underlying mechanisms[J]. Cells,2021,10(8):1958.
[4] AFSAR B,ELSURER R. Increased renal resistive index in type 2 diabetes:clinical relevance,mechanisms and future directions[J]. Diabetes Metab Syndr,2017,11(4):291-296.
[5] LV Z Q,GUO Y J. Metformin and its benefits for various diseases[J]. Front Endocrinol,2020,11:191.
[6] SONG A N,ZHANG C,MENG X F. Mechanism and application of metformin in kidney diseases:an update[J]. Biomed Pharmacother,2021,138:111454.
[7] LAMOIA T E,SHULMAN G I. Cellular and molecular mechanisms of metformin action[J]. Endocr Rev,2021,42(1):77-96.
[8] EIBL G,ROZENGURT E. Metformin:review of epidemiology and mechanisms of action in pancreatic cancer[J]. Cancer Metastasis Rev,2021,40(3):865-878.
[9] HAN Y C,TANG S Q,LIU Y T,et al. AMPK agonist alleviate renal tubulointerstitial fibrosis via activating mitophagy in high fat and streptozotocin induced diabetic mice[J]. Cell Death Dis,2021,12(10):925.
[10] AGIUS L,FORD B E,CHACHRA S S. The metformin mechanism on gluconeogenesis and AMPK activation:the metabolite perspective[J]. Int J Mol Sci,2020,21(9):3240.
[11] MA T,TIAN X,ZHANG B D,et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2[J]. Nature,2022,603(7899):159-165.
[12] FORETZ M,GUIGAS B,VIOLLET B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus[J]. Nat Rev Endocrinol,2019,15(10):569-589.
[13] HUA Y,ZHENG Y,YAO Y R,et al. Metformin and cancer hallmarks:shedding new lights on therapeutic repurposing[J]. J Transl Med,2023,21(1):403.
[14] XIAO N,WANG J,WANG T,et al. Metformin abrogates pathological TNF-α-producing B cells through mTOR-dependent metabolic reprogramming in polycystic ovary syndrome[J]. Elife,2022,11:e74713.
[15] MOHAMMAD H M F,GALAL GOUDA S,ELADL M A,et al. Metformin suppresses LRG1 and TGFβ1/ALK1-induced angiogenesis and protects against ultrastructural changes in rat diabetic nephropathy[J]. Biomed Pharmacother,2023,158:114128.
[16] PUGLIESE G,PENNO G,NATALI A,et al. Diabetic kidney disease:new clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on the natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function[J]. J Nephrol,2020,33(1):9-35.
[17] FADDEN E J,LONGLEY C,MAHAMBREY T. Metformin-associated lactic acidosis[J]. BMJ Case Rep,2021,14(7):e239154.
[18] MARIANO F,BIANCONE L. Metformin,chronic nephropathy and lactic acidosis:a multi-faceted issue for the nephrologist[J]. J Nephrol,2021,34(4):1127-1135.
[19] MAURO S D,FILIPPELLO A,SCAMPORRINO A,et al. Metformin:when should we fear lactic acidosis?[J]. Int J Mol Sci,2022,23(15):8320.
[20] RAJASURYA V,ANJUM H,SURANI S. Metformin use and metformin-associated lactic acidosis in intensive care unit patients with diabetes[J]. Cureus,2019,11(5):e4739.
[21] THAMMAVARANUCUPT K,PHONYANGNOK B,PARAPIBOON W,et al. Metformin-associated lactic acidosis and factors associated with 30-day mortality[J]. PLoS One,2022,17(8):e0273678.
[22] ALVAREZ C A,HALM E A,PUGH M J V,et al. Lactic acidosis incidence with metformin in patients with type 2 diabetes and chronic kidney disease:a retrospective nested case-control study[J]. Endocrinol Diabetes Metab,2021,4(1):e00170.
[23] TRINKLEY K E,ANDERSON H D,NAIR K V,et al. Assessing the incidence of acidosis in patients receiving metformin with and without risk factors for lactic acidosis[J]. Ther Adv Chronic Dis,2018,9(9):179-190.
[24] OSHIMA M,SHIMIZU M,YAMANOUCHI M,et al. Trajectories of kidney function in diabetes:a clinicopathological update[J]. Nat Rev Nephrol,2021,17(11):740-750.
[25] LIU P,ZHANG Z D,LI Y. Relevance of the pyroptosis-related inflammasome pathway in the pathogenesis of diabetic kidney disease[J]. Front Immunol,2021,12:603416.
[26] SAMSU N. Diabetic nephropathy:challenges in pathogenesis,diagnosis,and treatment[J]. Biomed Res Int,2021,2021:1497449.
[27] GUO J,ZHENG H J,ZHANG W T,et al. Accelerated kidney aging in diabetes mellitus[J]. Oxid Med Cell Longev,2020,2020:1-24.
[28] PEREIRA P R,CARRAGETA D F,OLIVEIRA P F,et al. Metabolomics as a tool for the early diagnosis and prognosis of diabetic kidney disease[J]. Med Res Rev,2022,42(4):1518-1544.
[29] JIN J,SHI Y F,GONG J G,et al. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte[J]. Stem Cell Res Ther,2019,10(1):95.
[30] SELBY N M,TAAL M W. An updated overview of diabetic nephropathy:diagnosis,prognosis,treatment goals and latest guidelines[J]. Diabetes Obes Metab,2020,22(Suppl 1):3-15.
[31] XUE J,WANG L,SUN Z X,et al. Basic research in diabetic nephropathy health care:a study of the renoprotective mechanism of metformin[J]. J Med Syst,2019,43(8):1-13.
[32] TYPIAK M,KULESZA T,RACHUBIK P,et al. Role of klotho in hyperglycemia:its levels and effects on fibroblast growth factor receptors,glycolysis,and glomerular filtration[J]. Int J Mol Sci,2021,22(15):7867.
[33] CHEN X W,TAN H S,XU J,et al. Klotho-derived peptide 6 ameliorates diabetic kidney disease by targeting Wnt/β-catenin signaling[J]. Kidney Int,2022,102(3):506-520.
[34] WANG Q,REN D J,LI Y B,et al. Klotho attenuates diabetic nephropathy in db/db mice and ameliorates high glucose-induced injury of human renal glomerular endothelial cells[J]. Cell Cycle,2019,18(6/7):696-707.
[35] TYPIAK M,KULESZA T,RACHUBIK P,et al. Role of klotho in hyperglycemia:its levels and effects on fibroblast growth factor receptors,glycolysis,and glomerular filtration[J]. Int J Mol Sci,2021,22(15):7867.
[36] XUE J,WANG L,SUN Z X,et al. Basic research in diabetic nephropathy health care:a study of the renoprotective mechanism of metformin[J]. J Med Syst,2019,43(8):266.
[37] AL-KURAISHY H M,AL-GAREEB A I,SAAD H M,et al. The potential effect of metformin on fibroblast growth factor 21 in type 2 diabetes mellitus(T2DM)[J]. Inflammopharmacology,2023,31(4):1751-1760.
[38] GU L Y,TANG H T,XU Z X. Huangkui capsule in combination with metformin ameliorates diabetic nephropathy via the Klotho/TGF-β1/p38MAPK signaling pathway[J]. J Ethnopharmacol,2021,281:113548.
[39] RENA G,HARDIE D G,PEARSON E R. The mechanisms of action of metformin[J]. Diabetologia,2017,60(9):1577-1585.
[40] REN H W,SHAO Y,WU C,et al. Metformin alleviates oxidative stress and enhances autophagy in diabetic kidney disease via AMPK/SIRT1-FoxO1 pathway[J]. Mol Cell Endocrinol,2020,500:110628.
[41] WANG W N,SUN W X,CHENG Y L,et al. Role of sirtuin-1 in diabetic nephropathy[J]. J Mol Med,2019,97(3):291-309.
[42] XU J,LIU L Q,XU L L,et al. Metformin alleviates renal injury in diabetic rats by inducing Sirt1/FoxO1 autophagic signal axis[J]. Clin Exp Pharmacol Physiol,2020,47(4):599-608.
[43] GAO Y Y,TIAN W,ZHANG H N,et al. Canonical transient receptor potential channels and their modulators:biology,pharmacology and therapeutic potentials[J]. Arch Pharm Res,2021,44(4):354-377.
[44] SZREJDER M,RACHUBIK P,ROGACKA D,et al. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions[J]. Biochim Biophys Acta Mol Basis Dis,2020,1866(3):165610.
[45] YANG H M,XIE T T,LI D R,et al. Tim-3 aggravates podocyte injury in diabetic nephropathy by promoting macrophage activation via the NF-κB/TNF-α pathway[J]. Mol Metab,2019,23:24-36.
[46] OPAZO-RÍOS L,PLAZA A,SÁNCHEZ MATUS Y,et al. Targeting NF-κB by the cell-permeable NEMO-binding domain peptide improves albuminuria and renal lesions in an experimental model of type 2 diabetic nephropathy[J]. Int J Mol Sci,2020,21(12):4225.
[47] ZHANG L,NIU J S,ZHANG X M,et al. Metformin can alleviate the symptom of patient with diabetic nephropathy through reducing the serum level of hcy and IL-33[J]. Open Med,2019,14:625-628.
[48] KANG Z F,ZENG J W,ZHANG T,et al. Hyperglycemia induces NF-κB activation and MCP-1 expression via downregulating GLP-1R expression in rat mesangial cells:inhibition by metformin[J]. Cell Biol Int,2019,43(8):940-953.
[49] PATIAL V,KATOCH S,CHHIMWAL J,et al. Tinospora cordifolia activates PPARγ pathway and mitigates glomerular and tubular cell injury in diabetic kidney disease[J]. Phytomedicine,2021,91:153663.
[50] ABDELKADER N F,IBRAHIM S M,MOUSTAFA P E,et al. Inosine mitigated diabetic peripheral neuropathy via modulating GLO1/AGEs/RAGE/NF-κB/Nrf2 and TGF-β/PKC/TRPV1 signaling pathways[J]. Biomed Pharmacother,2022,145:112395.
[51] TANG D,HE W J,ZHANG Z T,et al. Protective effects of Huang-Lian-Jie-Du Decoction on diabetic nephropathy through regulating AGEs/RAGE/Akt/Nrf2 pathway and metabolic profiling in db/db mice[J]. Phytomedicine,2022,95:153777.
[52] ISHIBASHI Y,MATSUI T,TAKEUCHI M,et al. Beneficial effects of metformin and irbesartan on advanced glycation end products(AGEs)-RAGE-induced proximal tubular cell injury[J]. Pharmacol Res,2012,65(3):297-302.
[53] 庞若宇,关美萍,郑宗基,等. 二甲双胍对糖基化终末产物诱导的成纤维细胞凋亡及相关蛋白caspase-3、Bax及Bcl-2表达的影响[J].南方医科大学学报,2015,5(6):898-902.
[54] ZHU L L,WANG H Y,TANG T. Effects of miR-195 on diabetic nephropathy rats through targeting TLR4 and blocking NF-κB pathway[J]. Eur Rev Med Pharmacol Sci,2021,25(3):1522-1529.
[55] XU J,XIANG P,LIU L Q,et al. Metformin inhibits extracellular matrix accumulation,inflammation and proliferation of mesangial cells in diabetic nephropathy by regulating H19/miR-143-3p/TGF-β1 axis[J]. J Pharm Pharmacol,2020,72(8):1101-1109.
[56] LIU S,WU W,LIAO J,et al. MicroRNA-21:a critical pathogenic factor of diabetic nephropathy[J]. Front Endocrinol(Lausanne),2022,13:895010.


